Bioavailability is dose-related
Curcuminoid concentrations
Turmeric is composed of various subgroups of active compounds; one of them defined as the “Curcuminoids”. Curcuminoids are only a small fraction of the turmeric rhizome.
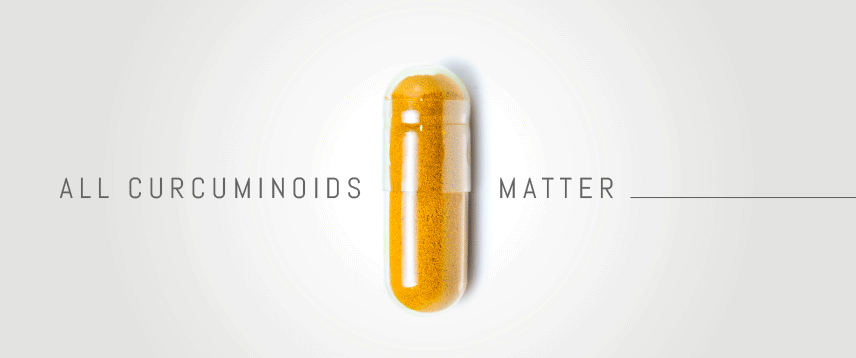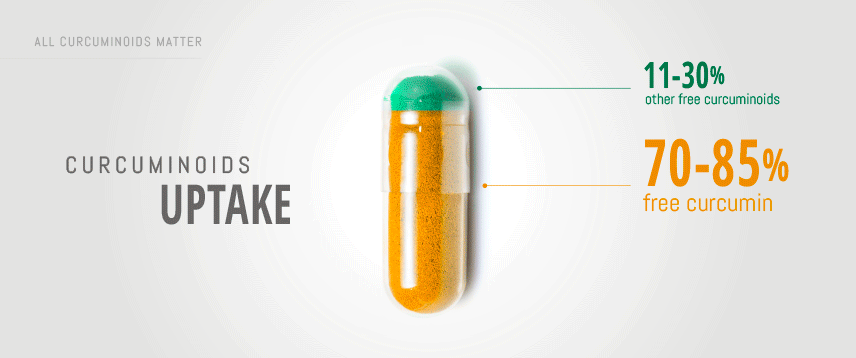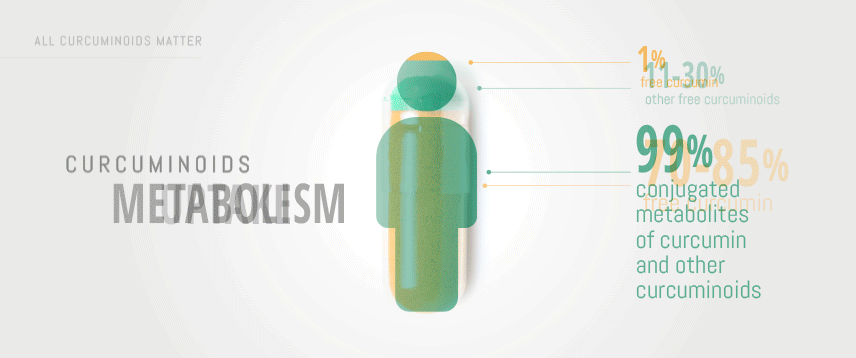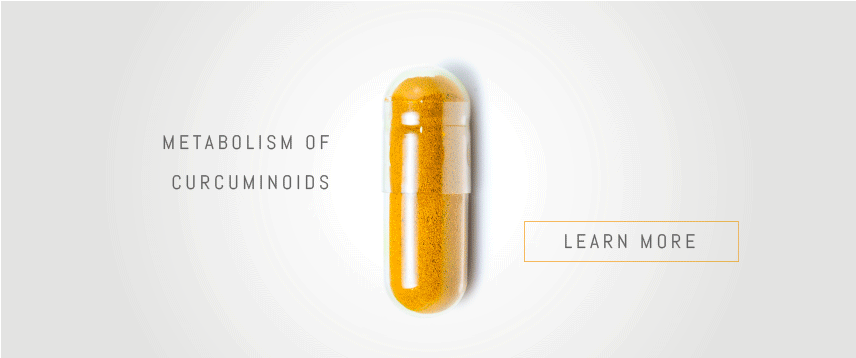
This sub-family of elements is composed of three polyphenolic pigments: curcumin, demethoxycurcumin and bisdemethoxycurcumin. It has been reported that the natural ratio is as following: 70-85% curcumin, 10-20% demethoxycurcumin and less than 1-10% bisdemethoxycurcumin. Therefore, only considering the mass of curcuminoids preparations ingested is not the right approach to optimize bioavailability and efficacy of curcumin.
The dose relationship between bioavailability and efficacy
Most commercially available turmeric preparations are standardized extracts (STE) containing 95% curcuminoids. Of these, 70-85% is curcumin and the rest are other parent curcuminoids, demethoxycurcumin (DMC) and bisdemethoxycurcumin (BDMC).
When considering absorption levels after ingestion, evidence from in vitro and in vivo studies shows that a high dose of STE 95% curcuminoids is required to achieve efficacy. Typically, the STE daily dosage used to observe a physiological effect is around 1500-2000mg, with or without piperine extract. Due to their limited bioavailability at a low dose, STE and many other turmeric formulations therefore necessitate high dose.
Naturex, part of Givaudan, has developed an enhanced formulation that offers high bioavailability. Curcuminoids are formulated in a water-dispersible dried colloidal suspension capable of improving bioavailability at a low dose.
As discussed previously, curcuminoids are poorly absorbed by the body. Therefore, multiple strategies have been used to improve bioactivity.
Avoiding bias in studies of curcuminoid bioavailability
To overcome the low availability of curcuminoids, pharmacokinetic studies conducted by companies tend to investigate the effect of a high dose of their formulation (higher than that used in real life), or to compare it with a low dose of a competitor’s formulation.
To avoid bias and to respect the minimal dose necessary to demonstrate bioequivalence, Naturex’s pharmacokinetic trial assessed total curcuminoid absorption by comparing a low dose of TurmiPure Gold® to the “recommended real-life” dose of STE and other studied turmeric preparations.
TurmiPure Gold® — Higher bioavailability and bioequivalence
TurmiPure Gold® is an enhanced turmeric extract formulation, which offers very high assimilation compared to commercially available turmeric preparations standardized to 95% curcuminoids. Multiple clinical studies support its various applications.
Each 300 mg capsule of TurmiPure Gold® contains 90 mg of curcuminoids (including 69 mg of curcumin) formulated in a patent pending water-dispersible colloidal suspension. This hydro-dispersible formulation allows curcuminoids to penetrate much more easily into the bloodstream and cells.
Using dose-related assessment, our pharmacokinetic study showed:
- Improved bioavailability, with an assimilation factor of 24
- Bioequivalence is used to compare and demonstrate biological equivalence in terms of the bioavailability or bioactivity of two substances. To reach the same level of curcuminoids in blood as 300mg of TurmiPure Gold®, you would need either 1922mg of a standard turmeric extract (95% curcuminoids (STE)), or 2264mg of a turmeric extract (95% curcuminoids-piperine combination (TEP))




