Evidence from our clinical pharmacokinetic study
Get access to the full published version here
THE STRONGEST COMPARATIVE HUMAN PHARMACOKINETIC STUDY TO DATE*
* 2021, Fança Berthon & all, Pharmacokinetics of a Single Dose of Turmeric Curcuminoids Depends on Formulation: Results of a Human Crossover Study, The Journal of Nutrition, Volume 151, Issue 7, July 2021, Pages 1802–1816, https://doi.org/10.1093/jn/nxab087
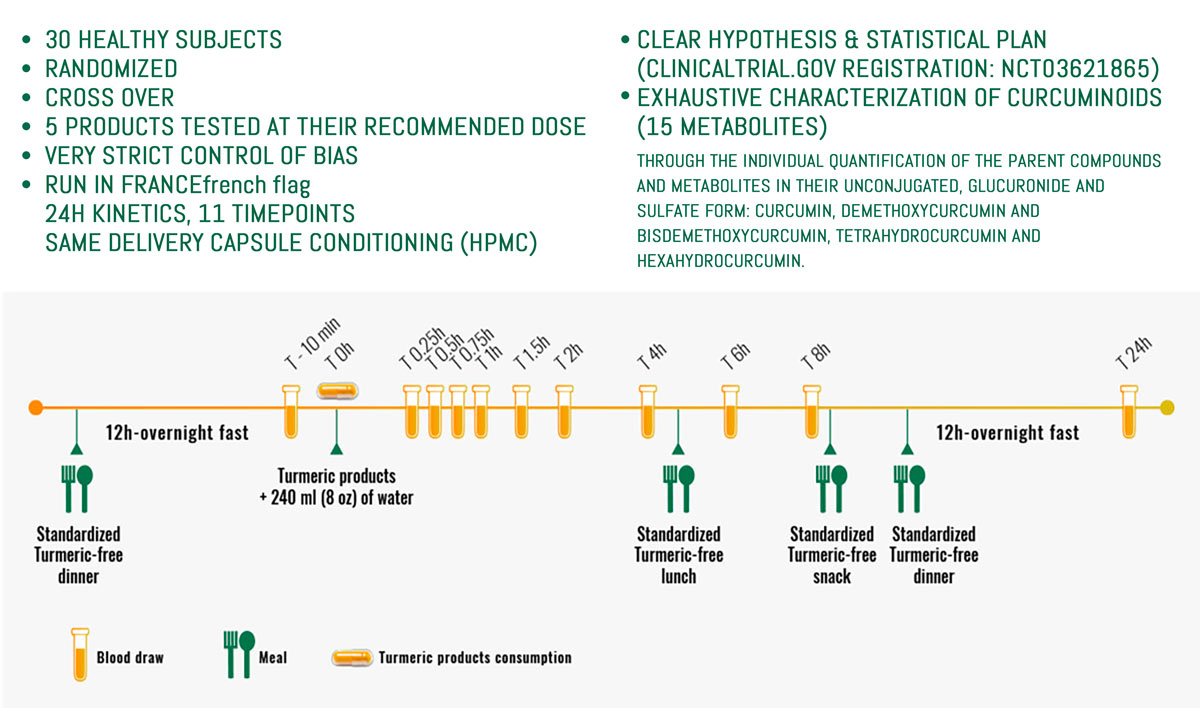
Evidence#1 – The smallest dose reaching significant curcuminoid absorption
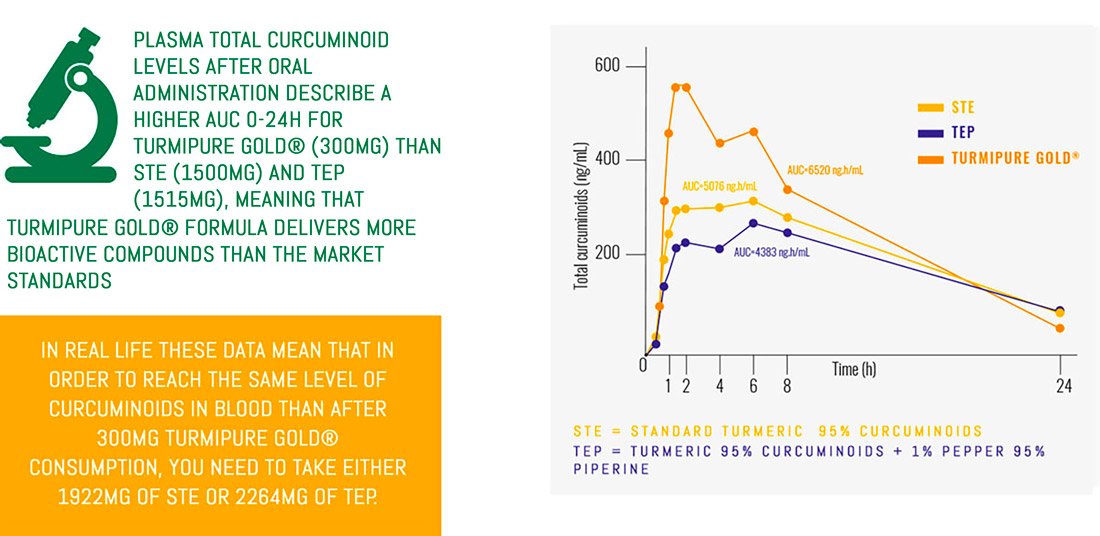
TurmiPure Gold® is the world’s first turmeric to clinically demonstrate bioequivalence at a low dose.
Bioequivalence is when two products are equivalent for a specific effect, for example the amount of curcuminoids absorbed by the body.
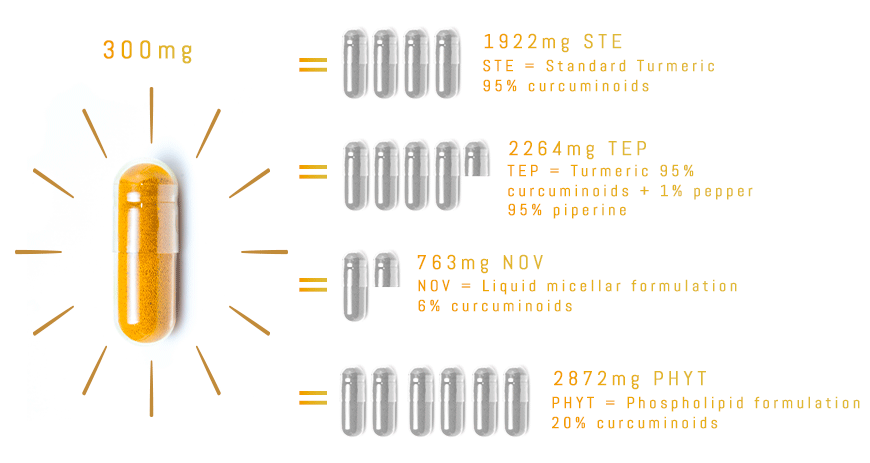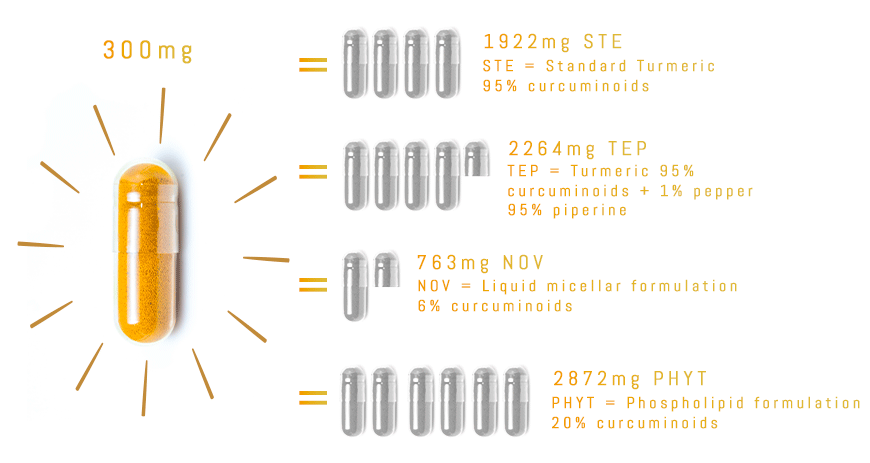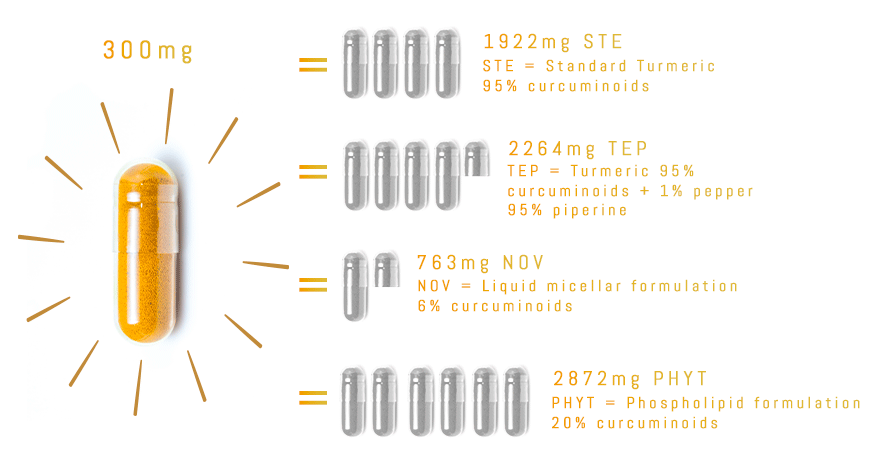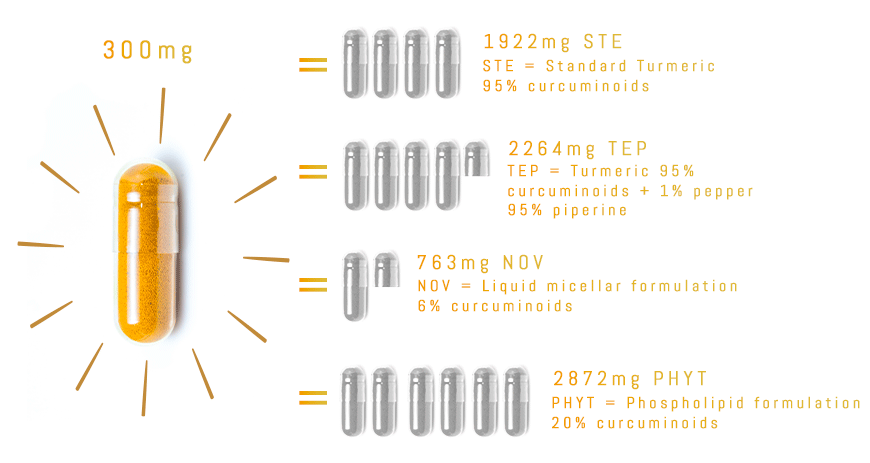
With TurmiPure Gold®, there is no need to take a high daily dose of turmeric. 300mg of TurmiPure Gold® delivers the same amount of curcuminoids as an effective high dose of market standards.
Evidence#2 – The most bioavailable turmeric at a low dose
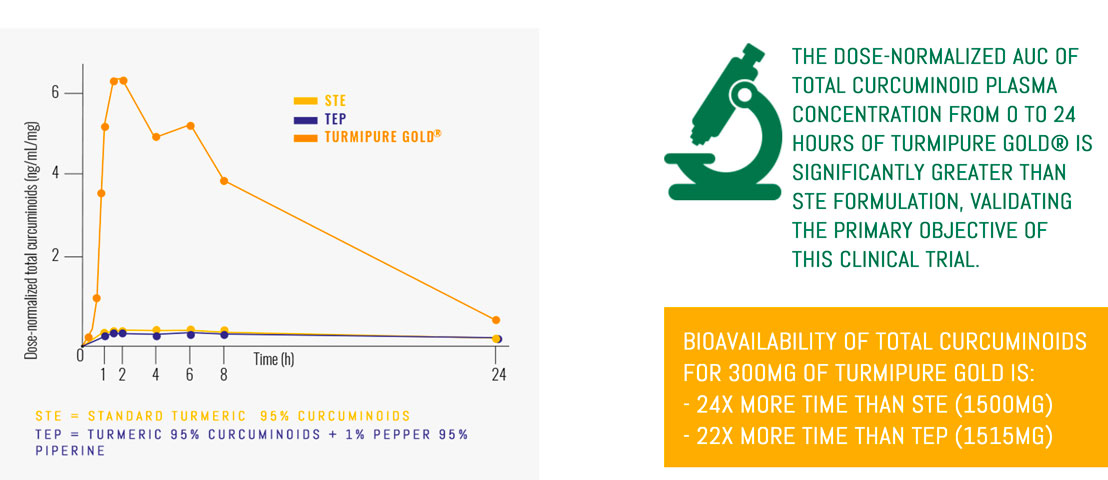
With a dose of only 300mg, TurmiPure Gold® enhances the bioavailability of total curcuminoids.
Bioavailability is the measurement of the rate and extent to which an active compound reaches the blood once it has been administered.
Curcuminoids, turmeric’s bioactive compounds, are poorly absorbed and rapidly eliminated by the body, which reduces their efficacy. Thus, improving curcuminoid absorption is a key challenge to maximizing the delivery of turmeric’s benefits…
With a dose of only 300mg, TurmiPure Gold® enhances the bioavailability of total curcuminoids:




